Background: Anti-CD19 chimeric antigen receptor T cell (CART19) immunotherapy is now standard treatment for large B cell lymphomas (LBCL), but its use is limited by high costs and requirement for access to specialized tertiary care centers. In this study, we sought to investigate whether minority health populations (MHP) have equal access to CART19 and similar outcomes compared to non-MHP patients (pts).
Methods: MHP was defined as self-reported non-White groups (not accounting for Hispanic/Latino ethnicity). We retrospectively evaluated three cohorts of non-Hodgkin lymphoma (NHL) pts from two Institution's catchment areas (defined as the counties providing at least 80% of an institution's pts) characterized by different proportions of MHP: a metropolitan area with a proportion of MHP higher than the US average (Abramson Cancer Center, ACC - Philadelphia, Pennsylvania) and a more rural area with a lower proportion of MHP (Knight Cancer Institute, KCI - Portland, Oregon).
The first cohort (catchment area NHL cohort) included pts diagnosed with NHL in the catchment area of each Institution between January 2015 and December 2019 (most recent cancer registry data available), accounting for 8,956 cases for ACC and 4,568 for KCI.
The second cohort (total LBCL cohort) included LBCL pts treated with any therapy at each Institution during the same timeframe as the third cohort (January 2018, when the first patient was treated with commercial CART19, and December 2022). The total LBCL cohort included 1,492 LBCL pts from ACC (1,111 from ACC catchment area) and 396 pts from KCI (388 from KCI catchment area). LBCL diagnosis was searched in the Electronic Medical Records using the following International Classification of Diseases (ICD) 10 definition: Diffuse Large B-cell Lymphoma (ICD-10-CM: C83.30-39) and Mediastinal (thymic) large B-cell Lymphoma (ICD-10-CM: C85.20-29).
The third cohort (CART19 LBCL cohort) included the subset of the pts in the total LBCL cohort treated with commercial CART19 at ACC (194 pts;132 from catchment area) and at KCI (47 pts; 45 from catchment area) between January 2018 and December 2022. The data collection cut-off date was March 31 st, 2023. Pts were evaluated for 3-month response (Lugano criteria), progression-free survival (PFS), overall survival (OS), and toxicities (ASTCT, CTCAE) in the 30 days after CART19 infusion.
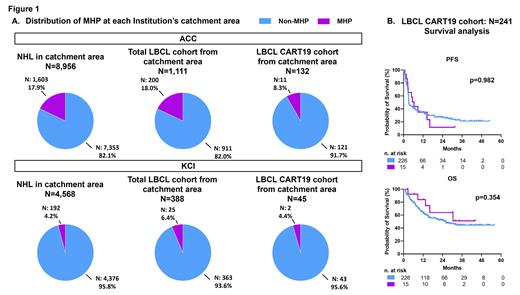
Results: In the ACC catchment area NHL cohort, the proportion of MHP was 17.9%. In the total LBCL cohort of pts treated at ACC, the proportion of MHP patients was similar to the catchment area NHL cohort (15.7%). However, in the ACC CART19 LBCL cohort, the proportion of MHP pts was 6.7%. By excluding patients from outside the catchment area, the proportion of MHP pts in the ACC total LBCL cohort was 18.0%, and 8.3% in the CART19 LBCL cohort (p=0.004). At KCI, the proportion of MHP in NHL catchment area cohort was, as expected, lower 4.2%. In the total LBCL KCI cohort, MHP was 6.6%and in the CART19 LBCL cohort was 4.2%, although the number of MHP pts in this cohort was very low (n=2). Most pts in the KCI group were from the catchment area ( Fig. 1A).
We then studied pts outcomes based on MHP status in the combined ACC and KCI CART19 LBCL cohorts (total: 241; non-MHP: 226; MHP: 15). Pts characteristics according to MHP status were balanced except for age at CART19 infusion (median age: MHP 50 vs non-MHP 62 years). MHP pts had a complete response rate (CRR) of 66.7% vs. 42.0% for non-MHP pts (p=0.104). The 12-month PFS was 34.9% for MHP and 34.9% for non-MHP pts (p=0.982). Likewise, no difference in OS was observed between the two groups (p=0.354) ( Fig. 1B). No difference in terms of the incidence and any grade CRS (66.7% vs. 63.3%; p=0.792) and ICANS (13.3% vs. 22.1%; p=0.423) was observed in MHP and non-MHP pts. To exclude that the outcome analysis was affected by the different age distributions in the two groups, we performed efficacy and toxicity analysis only in the population younger than 60 years old (n=107, MHP=12, non-MHP=95). Pts characteristics were similar in the two groups. CRR, PFS, OS, and toxicities did not differ between the two groups.
Conclusion: This study shows that MHP access to tertiary care for LBCL is equitable, but reduced for commercial CART19 immunotherapy. Nevertheless, when treated with CART19, MHP pts have similar survival and safety outcomes compared to non-MHP. This study supports further efforts to establish equitable access to CART19 immunotherapy for diverse populations.
Disclosures
Ghilardi:viTToria biotherapeutics: Consultancy. Nemecek:Novartis: Consultancy. Barta:Janssen: Consultancy; Acrotech: Consultancy; Affimed: Consultancy; Daiichi Sankyo: Consultancy. Svoboda:Astra Zeneca: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; TG Therapeutics: Research Funding; BMS: Consultancy, Research Funding; Atara: Consultancy; ADCT: Consultancy; Genmab: Consultancy; Merck: Research Funding; Pharmacyclics: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; SEAGEN: Consultancy, Research Funding. Chong:MJH Healthcare Holdings, LLC: Honoraria; Genentech: Research Funding; Abbvie: Research Funding; Novartis: Honoraria; BMS: Honoraria; Beigene: Honoraria. Landsburg:Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Travel funding; Curis: Research Funding; Calithera: Membership on an entity's Board of Directors or advisory committees, Research Funding; Epizyme: Membership on an entity's Board of Directors or advisory committees; ADCT: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees. Chen:Elsevier: Consultancy; Kite: Consultancy, Research Funding; Intellia: Consultancy; Fate: Research Funding; Novartis: Research Funding. Porter:Wiley and Sons Publishing: Honoraria; Sana Therapeutics: Consultancy, Current equity holder in publicly-traded company; Tmunity: Patents & Royalties; Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Mirror Biologics: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Genentech: Current equity holder in publicly-traded company; DeCart: Membership on an entity's Board of Directors or advisory committees; Capstan Bio: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Angiocrine Bio: Membership on an entity's Board of Directors or advisory committees. Garfall:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety and Monitoring Board; BMS: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Legend: Consultancy, Honoraria. Maziarz:Athersys: Other: Patent holder; Orca Therapeutics: Research Funding; Gamida: Research Funding; AlloVir: Consultancy, Research Funding; Kite: Consultancy; Novartis: Consultancy, Research Funding. Schuster:Novartis: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Morphosys: Consultancy; MustangBio: Consultancy; Genentech/Roche: Consultancy, Research Funding; Janssen: Consultancy; Nordic: Consultancy; Regeneron: Consultancy; Legend Biotech: Consultancy; Loxo: Consultancy; Acerta: Consultancy; BiGene: Consultancy; Celgene: Consultancy, Research Funding; Nanovecter: Consultancy; Pharmacyclics: Consultancy; Merck: Research Funding; DTRM: Research Funding; Juno Therapeutics: Research Funding; Abbvie: Research Funding; Adaptive Biotechnologies: Research Funding; TG Therapeutics: Research Funding. Ruella:NanoString: Consultancy, Research Funding; Bayer: Consultancy; Beckman Coulter: Research Funding; AbClon: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; viTToria biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Founder, Research Funding; GlaxoSmithKline: Consultancy.